Nitrogen exists in many forms in the soil and is essential for plant growth and development. Most of the nitrogen found in the soil is in numerous different organic forms. However, plants are unable to use organic forms of nitrogen. Normal, rather complex processes in the soil convert nitrogen from organic forms into ammonium.
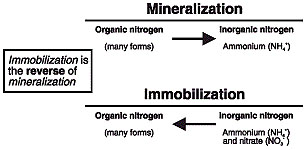 Figure 1
Figure 1
Mineralization and immobilization processes
What affects mineralization and immobilization?
Decaying plant material, humus and/or organic matter in the soil are important sources of nitrogen. How much plant residue or organic matter are in the soil, and the types present, can affect the types of nitrogen conversion processes that take place. For example, the presence of a high amount of wheat straw or corn stalks in the soil will result in immobilization processes taking place. Immobilization results in plant usable forms of nitrogen in the soil becoming unavailable for subsequent crop growth. This nitrogen is used by microorganisms in the decomposition process of wheat straw or corn stalks. Once the wheat straw or corn stalks have become highly decayed, immobilization stops and mineralization starts. That is, plant usable forms of nitrogen such as ammonium become available again. Immobilization processes generally do not take place when a legume crop, such as alfalfa or white clover, is plowed under. Mineralization will likely be the dominant process.
Conversion processes of nitrogen to different forms are all accomplished by various groups and types of microorganisms found in the soil. Factors that affect microorganisms, such as temperature, oxygen supply and moisture, can affect the degree to which nitrogen conversions take place.
Impact on water quality
Both immobilization and mineralization processes can have a direct impact on water quality. Immobilization can result in a reduction of inorganic forms of nitrogen, including nitrate. However, this reduction in nitrate is generally temporary.
Mineralization results in the production of ammonium (NH+). Under favorable conditions, ammonium is further converted by microorganisms to nitrate. Buildup of nitrates in soil followed by heavy rains can move plant available nitrate below the root zone. Nitrate is a form of nitrogen that is highly mobile and easily moves with water.
Whether nitrates continue to move downward and into groundwater depends on underlying soil and/or bedrock conditions as well as depth to groundwater. If depth to groundwater is shallow and the underlying soil is sandy, the potential for nitrates to enter groundwater is relatively high. However, if depth to groundwater is deep and the underlying soil is heavy clay, groundwater contamination from nitrates is not likely.
Once nitrates get into the groundwater, the greatest concerns are for infants less than one year old and for young or pregnant animals. High levels of nitrates can be toxic to newborns, causing anoxia, or internal suffocation. Seek alternative water sources if nitrate levels exceed the health standard of 10 ppm nitrate-N. Do not boil water to eliminate nitrates. It increases nitrate levels rather than decreasing them. The most common symptom of nitrate poisoning in babies is a bluish color to the skin, particularly around the baby's eyes and mouth. These symptoms of nitrate toxicity are commonly referred to as the "blue-baby" syndrome.
The material is based upon work supported by the United States Department of Agriculture, Extension Service, under special project number 89-EWQI-1-9203.