Many organisms live in the soil. Some of these are able to change ammonium nitrogen (NH4+) to nitrate nitrogen (NO3-). This process is called nitrification. Nitrification has two steps — both are carried out by bacteria that live in the soil (Figure 1). Common sources of ammonium in the soil result from decaying plants and organic matter, or ammonium can come from the application of manure or nitrogen fertilizers.
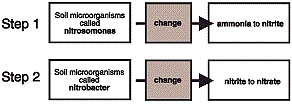 Figure 1
Figure 1
The nitrification process
When does this happen?
Nitrification depends on microorganisms. Factors such as organic matter, water content, oxygen supply, temperature and soil pH can affect how much, or how little, ammonium (NH4+) is converted to nitrate (NO3-). Warm, moist soils with good oxygen supply provide favorable conditions for nitrification. Practically speaking, nitrification is very active during the spring and summer months, slows in the fall, and is essentially nonexistent during the winter.
Impact on water quality
The nitrification process can have a direct impact on water quality. The end result of nitrification, nitrate (NO-), is a form of nitrogen that is highly mobile and easily moves with water. When rainfall is heavy, nitrates can move downward in the soil. Whether nitrates continue to move downward, and into ground water, depends on underlying soil and/or bedrock conditions, as well as the depth to the ground water itself. If the depth to ground water is shallow and the underlying soil is sandy, the potential for nitrates to enter ground water is relatively high. However, if the depth to the ground water is deep and the underlying soil is heavy clay, ground water contamination from nitrates is not likely.
Nitrates that do enter ground water can originate from a variety of sources. Good management practices can reduce the potential of nitrates from animal manure and nitrogen fertilizers getting into the ground water. Applying manure and nitrogen fertilizers when crops are actively growing, and using nitrates for growth and development, will reduce the amount of nitrate in the soil system. That reduces the amount that could potentially be moved into groundwater. However, little can be done to minimize the movement of nitrates into groundwater that result from the ongoing decay of organic matter in the soil. The presence of nitrates in the soil can simply be the result of natural bacterial decay of organic matter.
Once nitrates get into the groundwater, the greatest concerns are for infants less than one year old and for young or pregnant animals. High levels of nitrates can be toxic to newborns, causing anoxia, or internal suffocation. Seek alternative water sources if nitrate levels exceed the health standard of 10 ppm nitrate-N. Do not boil water to eliminate nitrates. It increases nitrate levels rather than decreasing them. The most common symptom of nitrate poisoning in babies is a bluish color to the skin, particularly around the baby's eyes and mouth. These symptoms of nitrate toxicity are commonly referred to as the "blue-baby" syndrome.
The initial draft of this publication was written by Karen DeFelice, former associate extension agronomist; Nyle Wollenhaupt, former state extension agronomist; and Daryl Buchholz, state extension agronomist. This material is based upon work supported by the United States Department of Agriculture, Extension Service, under special project number 89-EWQI-1-9203.