We value clean water for many reasons. We depend on lakes and streams for drinking water and recreation. Wildlife depends on habitats created by healthy streams, wetlands and lakes. There is strong support in both rural and urban communities for efforts to maintain and improve water quality.
Water quality has declined in some parts of Missouri in the 1990s. The effect of agriculture — particularly the effect of phosphorus from agriculture — in surface water quality has become a focal point for controversy.
Does phosphorus reduce water quality? Is the problem nitrogen or phosphorus? What fields and practices cause high phosphorus in agricultural runoff? How can farmers protect our water quality from excess phosphorus? This guide demonstrates the complexities of reducing losses of phosphorus from agricultural land and the range of options available to help reach water quality goals.
Surface water quality
Nitrogen (N), phosphorus (P) and potassium (K), as essential macronutrients, are required for growth by all animals and plants. Lack of these nutrients can restrict growth. Farmers regularly apply fertilizers containing N, P and K to crops to increase yield.
Similarly, nutrient levels in surface water often restrict the growth of aquatic plant species. In freshwaters such as lakes and streams, phosphorus is typically the nutrient limiting growth, though occasionally nitrogen is the most limiting nutrient. Potassium is not a limiting element in water, so water quality concerns focus on nitrogen and phosphorus.
Increasing the amount of nutrients entering a stream or lake will increase the growth of aquatic plants and other organisms. Although these nutrients are necessary, excessive levels overstimulate the lake or stream, reducing the quality of the water. The progressive deterioration of water quality from overstimulation by nutrients is called eutrophication.
Eutrophication
As nutrient concentrations increase, surface water quality is degraded through the process of eutrophication. The following sequence characterizes changes in surface water quality with increasing nutrient concentration:
- Increased algae growth
- Reduced water clarity
- Water treatment problems
- Odor and bad taste
- Increased filtration costs
- Disinfectant byproducts with potential human health effects
- Reduced oxygen in the water
- Altered fisheries
- Fish kills
- Toxins from cyanobacteria (blue-green algae) affecting human and animal health
Once a stream or lake has excess phosphorus, it takes time to improve water quality. Excess phosphorus cycles between the bottom sediments and the water long after the source of excess phosphorus has been eliminated. Consequently water quality efforts must focus on prevention.
Missouri's water resources
All water resources were not created equal in Missouri. The Ozarks are known for their clear streams and lakes (defined as oligotrophic in Table 1). Other regions of the state typically have murkier water. These differences are a function of the geology and land use in the region. The Ozarks are dominated by forest and pasture on an old geologic landscape low in phosphorus. Much of the rest of Missouri has a greater percentage of agricultural land on a geological landscape that naturally supports higher phosphorus concentrations in water.
All Missouri's water resources can be impaired by excess nutrients from agricultural fields. However, the changes in water quality are more rapidly apparent in poorly nourished, oligotrophic water. A small increase in phosphorus concentration in these water bodies creates a dramatic decrease in water clarity. (See the discussion on Table Rock Lake, Figure 1.)
Table 1
Definitions for trophic status of fresh water based on phosphorus concentration and the distribution of Missouri reservoirs among trophic levels in the Ozarks and the balance of Missouri. Source: After Jones and Knowlton (1993).
| Trophic status | Definition, characteristics | Missouri distribution | |
|---|---|---|---|
| Ozark region | Rest of Missouri | ||
| Oligotrophic | Poorly nourished, clear waters with limited sediment and biological activity. Total P concentration 3/4 10 ppb | 100 percent | 0 percent |
| Mesotrophic | Intermediate productivity and clarity. Total P concentration 10 to 25 ppb | 45 percent | 55 percent |
| Eutrophic | High nutrient content. Potential water treatment problems with taste and odor. Total P concentration 25 to 100 ppb | 10 percent | 90 percent |
| Hypereutrophic | Highly productive, murky waters. Can support cyanobacteria that may produce toxins. Total P concentration > 100 ppb | 0 percent | 100 percent |
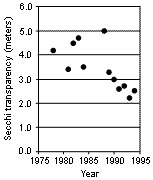 Figure 1
Figure 1
Decreasing clarity of Table Rock Lake between 1978 and 1994 as measured by secchi transparency. (From Jack Jones, MU.) Water quality decreased in Table Rock Lake between 1978 and 1994; some improvement has been observed since 1994. The secchi meter is a simple measure of water clarity based on the depth a disk can be viewed under water. Water clarity decreased by 2 meters (about 6.5 feet) over the 16-year period. The decrease in water clarity was associated with increasing total phosphorus concentration in the water
How is phosphorus lost from agricultural fields?
Phosphorus is carried in runoff water from agricultural fields into streams, wetlands and lakes. Phosphorus can travel attached to particles of soil or manure eroded by water into a stream (Figure 2). Phosphorous also can dissolve into runoff water as it passes over the surface of the field.
There is little potential for phosphorus to leach through soil into groundwater. Soil particles have a large capacity to fix phosphorus in forms that are immobile in soil. Most soils filter out soluble phosphorus as water passes through the soil profile into groundwater. However, this filtration process can be overloaded or bypassed under certain conditions, allowing higher concentrations of phosphorus into groundwater. Cracking soils or areas with karst topography create channels in the soil that allow surface water to travel directly to groundwater. The capacity of soil to adsorb phosphorus can be overwhelmed on sandy soils or when the water table is close to the soil surface.
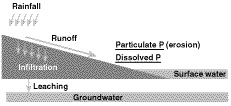 Figure 2
Figure 2
Potential pathways for phosphorus loss from agricultural fields
Phosphorus losses in runoff
Phosphorus losses from agricultural fields can be divided into three categories:
- Flash losses of soluble phosphorus soon after application of manure or fertilizer
- Slow leak losses of soluble phosphorus
- Erosion events.
Flash losses of soluble phosphorus
Manure and fertilizer have vastly higher concentrations of soluble phosphorus than soil. If a rainfall event causing runoff occurs soon after a surface application, the concentration of soluble phosphorus in the runoff can be more than 100 times higher than normal.
Over time, highly soluble manure and fertilizer phosphorus on the soil surface will react with the soil reducing soluble phosphorus in runoff back to initial levels. Normal levels return over the course of a month in warm soils, but this process takes longer in cold soils. Manure and fertilizer application is not recommended on frozen or snow-covered soils because phosphorus never has a chance to react with the soil before runoff occurs.
Research from Arkansas on poultry litter and swine manure applied to pastures shows that soluble phosphorus concentrations increase in direct proportion to increasing application rate in these flash phosphorus loss events.
Flash soluble phosphorus losses have high concentrations of phosphorus in a form that is readily available to aquatic organisms. These events occur with runoff soon after a surface application of phosphorus or when phosphorus is surface applied to frozen or snow-covered fields. However, one ill-timed application can contribute more phosphorus to surface water than is lost by all other processes over the course of a year or more.
To minimize flash soluble phosphorus losses:
- Apply phosphorus sources below the surface.
- Surface-apply phosphorus sources during periods of the year when runoff is unlikely.
- Surface-apply phosphorus sources only on fields with a low potential for runoff.
- Do not surface-apply phosphorus sources to frozen or snow-covered soils.
- Maintain buffer strips around water resources where no phosphorus is applied.
- Add alum or a similar treatment to manure to reduce the availability of phosphorus.
Slow leak losses of soluble phosphorus
All soils naturally release some soluble phosphorus into surface runoff. The concentration of soluble phosphorus in runoff is affected by the soil test phosphorus level of the soil.
Soil tests for phosphorus were developed to help estimate phosphorus fertilizer requirements for crops. Research on soils from other states indicate that soils near optimum soil test levels for growing crops typically supports soluble phosphorus concentrations of 0.5 ppm or less.
Considerable evidence suggests that soluble phosphorus concentration in runoff increases in direct proportion to increasing soil test phosphorus levels. This linear relationship changes from soil to soil. Tripling soil test phosphorus above the high soil test category may increase soluble phosphorus in runoff to 0.5-2.5 ppm.
To minimize slow leak soluble phosphorus loss"
- Apply phosphorus only to fields that have an agronomic need for phosphorus.
- Reduce the amount of annual runoff from agricultural fields through crop selection and soil conservation practices.
- Maintain buffer strips around water resources where no phosphorus is applied.
Erosion losses
When runoff water gains sufficient energy to cause soil erosion, the amount of phosphorus lost from the field increases dramatically. Reducing erosion losses through reduced or no-till on corn or wheat can reduce total phosphorus losses by 50 percent or more.
In soil, total phosphorus is much higher than the soluble phosphorus content. Soil particles have a tremendous capacity to fix soluble phosphorus allowing only a small proportion of the total and plant-available phosphorus to exist in the soluble form.
The natural sorting of soil particles during erosion causes those with the highest phosphorus concentration to be carried with runoff. Soils with higher soil test phosphorus levels will have higher phosphorus content in eroded particles.
To minimize erosion losses of phosphorus:
- Adopt soil conservation practices to minimize soil erosion.
- Maintain buffer strips around water resources where no phosphorus is applied.
Managing phosphorus losses in watersheds
Reducing the quantity of phosphorus reaching streams, wetlands and lakes will lead to long-term improvements in water quality of impaired lakes and streams.
Transferring phosphorus loss from one field in the watershed to another may not reduce the amount of phosphorus reaching a stream or lake. This is of particular importance to farmers applying manure. Reducing the rate of manure by half but covering twice the area within a watershed may not reduce the amount of phosphorus reaching the stream. If runoff is more likely on the additional land receiving manure, losses could be greater than the full rate on a field with lower runoff potential.
The quantity of phosphorus reaching a freshwater lake or stream is the product of the volume of runoff times the runoff phosphorus concentration:
P in runoff = (quantity of runoff) x (P concentration)
The quantity of phosphorus in surface runoff can be lowered either by reducing runoff quantity or by reducing the phosphorus concentration of runoff.
Farmers who apply phosphorus should adopt practices that limit runoff from their fields soon after application. High-testing soils should be cropped and tilled in a manner to minimize runoff and erosion.
Buffers between agricultural fields and water resources are a key component of lowering phosphorus concentrations.
Good management requires balancing conflicting objectives. For example, incorporating phosphorus into the soil eliminates flash phosphorus losses and may reduce soil test phosphorus levels on the surface, reducing slow leak losses. Tillage associated with incorporation may promote erosion, which increases the potential for phosphorus loss. Banding the phosphorus source while following the contour of the land will incorporate the phosphorus source with little increase in erosion potential.
Extensive tillage should never be used to lower surface soil test phosphorus, particularly on highly erodable land. Erosion losses from tillage will be much more damaging to the water resource than a high-testing soil surface with little erosion.
Phosphorus in groundwater
Phosphorus is primarily a surface water quality issue. The ability of soil particles to adsorb soluble phosphorus limits the movement of phosphorus through soil. Soil particles strip soluble phosphorus compounds from the water as it leaches through the soil profile. Phosphorus levels in soil leachate can be 10 percent of surface runoff concentrations.
Most Missouri soils have a tremendous capacity to adsorb phosphorus, particularly the highly weathered soils in the Ozark region.
Is only agriculture to blame?
There are many sources of phosphorus in the landscape. Impaired water can be affected by point sources of phosphorus such as industrial effluent and wastewater treatment plants and by nonpoint sources such as agricultural fields, urban runoff and septic systems.
Agriculture is the dominant land use in Missouri as nearly 60 percent of the land in the state is used in some aspect of agricultural production. Studies in Missouri and other states find the lowest water quality in watersheds dominated by agriculture.
In any watershed the contribution of agriculture versus other sources of phosphorus will depend on the relative mix of sources and activities in the watershed. For example, in the Table Rock Lake watershed, the wastewater treatment plant for the city of Springfield, the third largest city in the state, empties into the James River, which flows into the lake. A boom in construction along the shore has likely increased erosion losses and contributions from septic systems. The poultry industry also has dramatically expanded in recent years. Conversion from timber to other agricultural uses may also be a factor. Apportioning the relative contribution of these various nonpoint phosphorus sources requires detailed study — a time-consuming and expensive process.
Efforts to protect water quality can be evaluated. Farmers must take the initiative in adopting practices that minimize the loss of phosphorus from their fields into streams, wetlands and lakes. This is the best way to ensure the trust of their neighbors.
Conclusions
Phosphorus is a critical element for crop production. Proper management can limit the amount of phosphorus reaching streams, wetlands and lakes from agricultural fields. The successful steward of land and water resources will use crop selection, soil conservation measures, nutrient management planning, knowledge of weather patterns and common sense to limit phosphorus loss from agricultural fields.
Related reading
- Daniel, T. C., A. N. Sharpley, R. Wedepol, and J. L. Lemunyon. 1994. Minimizing surface water eutrophication from agriculture by phosphorus management. Journal of Soil and Water Conservation 49:30 to 38.
- Jones, J. R., and M. F. Knowlton. 1993. Limnology of Missouri reservoirs: An analysis of regional patterns. Lake and Reservoir Management 8:17 to 30.
- Sharpley, A. N., S. C. Chapra, R. Wedepold, J. T. Sims, T. C. Daniel, and K. A. Reddy. 1994. Managing agricultural phosphorus for protection of surface waters: Issues and options. Journal of Environmental Quality 23:437 to 451.