Once a pesticide is introduced into the environment, whether through an application, a disposal or a spill, it is influenced by many processes. These processes determine a pesticide's persistence and movement, if any, and its ultimate fate.
The fate processes can be beneficial. They can move a pesticide to the target area or destroy its potentially harmful residues. Sometimes they can be detrimental, leading to reduced control of a target pest, injury of nontarget plants and animals, and environmental damage. Of particular concern today is the movement of pesticides into groundwater.
Different soil and climatic factors and different handling practices can promote or prevent each process. An understanding of the fate processes can help every pesticide applicator ensure that applications are not only effective, but are also environmentally safe.
The fate processes
Fate processes fall into three major types: adsorption, transfer, and degradation (Figure 1).
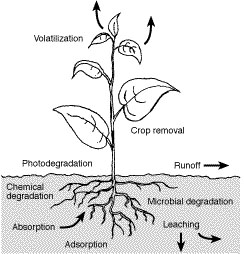 Figure 1
Figure 1
The three major fate processes of pesticides in the environment are adsorption, transfer and degradation.
Pesticide adsorption
The adsorption process binds pesticides to soil particles, similar to iron filings or paper clips sticking to a magnet. Adsorption often occurs because of the attraction between a chemical and soil particles. Positively charged pesticide molecules, for example, are attracted to and can bind to negatively charged clay particles.
Many soil factors influence pesticide adsorption. Soils high in organic matter or clay are more adsorptive than coarse, sandy soils, in part because a clay or organic soil has more particle surface area, or more sites onto which pesticides can bind. Moisture also affects adsorption. Wet soils tend to adsorb less pesticide than dry soils because water molecules compete with the pesticide for the binding sites.
Pesticides vary in their adsorption to soil particles. Some pesticides such as paraquat and glyphosate bind very tightly, while others bind only weakly and are readily desorbed or released back into the soil solution.
One problem resulting from pesticide adsorption is reduced pest control. For example, weeds may not be controlled if a herbicide is held tightly to soil particles and cannot be taken up by the roots of the target weeds. Some pesticide labels recommend higher application rates when the chemical is applied to adsorptive soils.
Plant injury can be another problem resulting from adsorption of pesticides to soil particles. Injury can result when a pesticide used for one crop is later released from the soil particles in amounts great enough to cause injury to a sensitive rotational crop. This pesticide ³carry-over² can also lead to the presence of illegal residues on rotational food or feed crops.
Adsorption is particularly important because it influences whether other processes are able to affect pesticides.
Pesticide transfer
Pesticide transfer is sometimes essential for pest control. For example, for certain preemergence herbicides to be effective, they must move within the soil to reach the germinating seeds. Too much movement, however, can move a pesticide away from the target pest. This can lead to reduced pest control, contamination of surface water and groundwater, and injury of nontarget species, including humans. Five ways that pesticides can be transferred are through volatilization, runoff, leaching, absorption and crop removal.
Volatilization
Volatilization is the conversion of a solid or liquid into a gas. Once volatilized, a pesticide can move in air currents away from the treated surface. Vapor pressure is an important factor in determining whether a pesticide will volatilize. The higher the vapor pressure, the more volatile the pesticide.
Environmental factors tend to increase volatilization. They include high temperature, low relative humidity, and air movement. A pesticide tightly adsorbed to soil particles is less likely to volatilize; soil conditions such as texture, organic matter content, and moisture can thus influence pesticide volatilization.
Volatilization can result in reduced control of the target pest because less pesticide remains at the target site. Vapor drift, the movement of pesticide vapors or gases in the atmosphere, can lead to injury of nontarget species. Herbicide vapors in particular can injure nontarget plants.
To reduce pesticide volatilization, avoid applying volatile pesticides when conditions are unfavorable, such as very hot, dry days or when the soils are wet. Labels often provide warnings if there is a volatility hazard under certain conditions.
Labels for volatile pesticides may suggest adding the pesticide to the soil by tillage or irrigation during or shortly after application. This helps to reduce volatilization by reducing the amount of exposed pesticide on the soil surface. Low-volatile formulations are also available for some pesticides.
Runoff
Runoff is movement of water over a sloping surface. Runoff occurs when water is applied faster than it can enter the soil. Pesticides can be carried in the water itself or bound to eroding soil particles.
The severity of pesticide runoff depends on the slope or grade of an area; the erodibility, texture and moisture content of the soil; and the amount and timing of rainfall and irrigation. Pesticide runoff usually is greatest when a heavy or sustained rain follows soon after an application. Over-irrigation can lead to excess surface water; it also can lead to pesticide runoff, especially when an irrigation system is used to apply a pesticide.
Vegetation or crop residue tends to slow the movement of runoff water. Certain physical and chemical properties of the pesticide, such as how quickly it is absorbed by plants or how tightly it is bound to plant tissue or soil, are also important.
Herbicide runoff can cause direct injury to nontarget plants. Insecticide and nematicide runoff into surface waters such as streams and ponds can be particularly harmful to aquatic organisms. Pesticide runoff also can lead to groundwater contamination and can cause injury to crops, livestock or humans if the contaminated water is used downstream.
Practices to reduce pesticide runoff include monitoring of weather conditions, careful application of irrigation water, using a spray mix additive to enhance pesticide retention on foliage, and incorporating the pesticide into the soil. Reduced-tillage cropping systems and surface grading, in addition to contour planting and strip cropping of untreated vegetation, can slow the movement of runoff water and help keep it out of wells, sinkholes, water bodies and other sensitive areas.
Leaching
Leaching is the movement of pesticides through the soil rather than over the surface. Leaching depends, in part, on the pesticide's chemical and physical properties. For example, a pesticide held strongly to soil particles by adsorption is less likely to leach. Another factor is solubility. A pesticide that dissolves in water can move with water in the soil. The persistence, or longevity, of a pesticide also influences the likelihood of leaching. A pesticide that is rapidly broken down by a degradation process is less likely to leach because it may remain in the soil only a short time.
Soil factors that influence leaching include texture and organic matter, in part because of their effect on pesticide adsorption. Soil permeability (how readily water moves through the soil) is also important. The more permeable a soil, the greater potential for pesticide leaching. A sandy soil is much more permeable than a clay.
The method and rate of application, the use of tillage systems that modify soil conditions, and the amount and timing of water a treated area receives after application can also influence pesticide leaching. Typically, the closer the time of application to a heavy or sustained rainfall, the greater the likelihood that some pesticide leaching will occur.
A certain amount of pesticide leaching may be essential for control of a target pest. Too much leaching, however, can lead to reduced pest control, injury of nontarget species and groundwater contamination.
Monitoring weather conditions and the amount and timing of irrigation can help minimize pesticide leaching. Careful pesticide selection is important because those pesticides that are not readily adsorbed, not rapidly degraded, and highly water soluble are the most likely to leach. Labels must be read carefully for instructions on the rates, timing and methods of application. The label may also advise against using the pesticide when certain soil, geologic or climatic conditions are present.
Pesticides can leach through the soil to groundwater from storage, mixing, equipment cleaning and disposal areas. Under certain conditions, some pesticides can leach to groundwater from normal applications. The section "Pesticides and water quality" provides further discussion on groundwater and safe handling practices to prevent contamination.
Absorption or uptake
Absorption or uptake is the movement of pesticides into plants and animals. Absorption of pesticides by target and nontarget organisms is influenced by environmental conditions and by the chemical and physical properties of the pesticide and the soil. Once absorbed by plants, pesticides may be broken down or they may remain in the plant until tissue decay or harvest.
Crop removal
Crop removal transfers pesticides and their breakdown products from the treatment site. Most harvested food commodities are subjected to washing and processing procedures that remove or degrade much of the remaining pesticide residue. While we typically associate harvesting with food and feed products, it is easy to forget that pesticides potentially can be transferred during such operations as tree and shrub pruning and turfgrass mowing.
Pesticide degradation
Pesticide degradation, or the breakdown of pesticides, usually is beneficial. Pesticide-destroying reactions change most pesticide residues in the environment to nontoxic or harmless compounds. However, degradation is detrimental when a pesticide is destroyed before the target pest has been controlled. Three types of pesticide degradation are microbial, chemical, and photodegradation.
Microbial degradation
Microbial degradation is the breakdown of pesticides by fungi, bacteria, and other microorganisms that use pesticides as a food source. Most microbial degradation of pesticides occurs in the soil. Soil conditions such as moisture, temperature, aeration, pH, and the amount of organic matter affect the rate of microbial degradation because of their direct influence on microbial growth and activity.
The frequency of pesticide application also is a factor that can influence microbial degradation. Rapid microbial degradation is more likely when the same pesticide is used repeatedly in a field. Repeated applications can actually stimulate the buildup of organisms that are effective in degrading the chemical. As the population of these organisms increases, degradation accelerates and the amount of pesticide available to control the pest is reduced. In extreme cases, accelerated microbial degradation has led to certain products being removed from the marketplace. Microorganisms greatly reduce the effectiveness of these chemicals soon after application.
The possibility of very rapid pesticide breakdown is reduced by using pesticides only when necessary and by avoiding repeated applications of the same chemical. Alternating between different classes, groups or formulations of pesticides can minimize the potential for microbial degradation problems as well as pest resistance.
Chemical degradation
Chemical degradation is the breakdown of pesticides by processes that do not involve living organisms. Temperature, moisture, pH and adsorption, in addition to the chemical and physical properties of the pesticide, determine which chemical reactions take place and how quickly they occur.
One of the most common pesticide degradation reactions is hydrolysis, a breakdown process in which the pesticide reacts with water. Many organophosphate and carbamate insecticides are particularly susceptible to hydrolysis under alkaline conditions. Some are actually broken down within a matter of hours when mixed with alkaline water.
Product labels may warn against mixing a pesticide with certain fertilizers, other pesticides or water with specific characteristics. Following these precautions can help prevent pesticide degradation and potential incompatibility problems. In some situations, buffers or other additives may be available to modify spray mix conditions and prevent or reduce degradation. Pesticide degradation and possible corrosion of application equipment can be avoided by not allowing a spray mix to remain in a tank for a long period of time.
Photodegradation
Photodegradation is the breakdown of pesticides by light, particularly sunlight. Photodegradation can destroy pesticides on foliage, on the surface of the soil, and even in the air.
Factors that influence pesticide photodegradation include the intensity of the sunlight, properties of the application site, the application method and the properties of the pesticide. Pesticide losses from photodegradation can be reduced by adding the pesticide to the soil during or immediately after application.
Pesticides and water quality
More than one-third of Missouri's population, including about 95 percent of the state's rural population, relies on groundwater as a source of drinking water. Most Missourians obtain their water from various surface water supplies. These water supplies are subject to contamination from many sources, including industrial and municipal waste, leaking underground fuel storage tanks, road salts, agricultural fertilizers and pesticides.
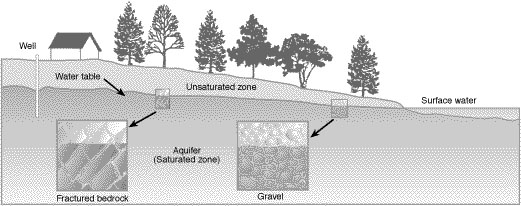 Figure 2
Figure 2
The groundwater system.
The groundwater system
Groundwater lies below the soil surface and fills the pore spaces in and around rock, gravel, sand and other materials. Contrary to popular belief, groundwater does not move through vast underground rivers and lakes, but through water-saturated zones called aquifers (Figure 2).
The upper level of an aquifer is called the water table. The water table level fluctuates throughout the year, lowering as water is removed from wells or discharged at streams and springs. The water table rises through recharge from rain and melting snow that seeps through soil into the aquifer.
For years it was believed that the natural filtering of water during its slow movement through the soil, sand, gravel and rock formations was adequate to cleanse it of contaminants before it reached groundwater. Today, many chemicals, including some pesticides, have been detected in groundwater. Studies have shown that recharge can carry pollutants down to aquifers. Furthermore, it seems clear that human activities can lead to contamination of the recharge water.
Not all groundwater is similarly vulnerable to contamination by pesticides. The deeper the water table is below the soil surface, the less likely that pollutants will reach groundwater. A deep aquifer provides more opportunities and time than does a shallow aquifer for pesticide adsorption, degradation and other processes to occur.
The permeability of the geologic layers between the soil surface and the groundwater is also important. If the materials above the water table are very coarse, such as sand or gravel or highly fractured rocks, water can move to groundwater more readily than if less permeable layers of clay or solid rock are present.
Bedrock such as limestone can make groundwater particularly prone to contamination, because it dissolves easily to form channels and depressions in the land surface. The depressions, called sinkholes, can provide a direct connection between the soil surface and the groundwater below. Contaminated water that drains into a sinkhole can readily enter groundwater, because the soil that lines the bottom of a sinkhole is often thin and provides little filtering of pollutants that enter.
Wells
A well is a direct conduit from the land surface to groundwater. The method of well construction, the frequency of well inspection and maintenance, and the proximity of a well to a pesticide source are important factors determining the potential for contamination.
Pesticides can reach groundwater by moving along the outside of the well casing or by entering an improperly capped or sealed well (Figure 3). Well casing forms the wall of a well. A cement compound of grout is forced into the space between the bore hole and the outside of the well casing to prevent water and contaminants from moving down along the outside of the casing into groundwater. Gravel, sand and other permeable materials are not adequate. The top of the casing is capped about 8 inches above the ground or at least high enough to prevent surface water from entering the top of the well.
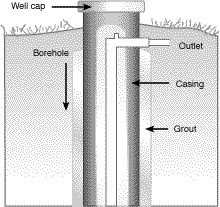 Figure 3
Figure 3
Cross section of upper portion of capped well.
Potential sources of contamination include sites used for storage, mixing, loading, disposal, or application of pesticides or where equipment is cleaned. Wells should be safe from both surface and subsurface contamination from such sources. Soil can be graded, or diversion terraces or ditches can be built upslope to intercept or divert surface runoff from the wellhead. Monitoring the area around a well can ensure that changes in land use do not increase the risk of contamination. Situating a well at least 10 feet from a building makes it accessible for maintenance and inspection.
Improperly closed or abandoned wells can contribute to groundwater contamination. The procedures for proper well closing can depend on site condition, but wells should be filled in such a way as to prevent water movement within the drill hole.
The surface water system
Surface water is water stored or flowing at the earth's surface: natural bodies of water such as rivers, lakes and wetlands, as well as constructed (artificial) water reservoirs such as canals, man-made lakes, and drainage ditches. The quantity and quality of surface water is important for many activities: consumption, recreation, transportation, waste assimilation, agricultural production and industrial use.
Surface water is linked to both groundwater and atmospheric water through the hydrologic cycle. Surface water moves into groundwater by infiltrating the soil and percolating downward; it also enters the atmosphere through evaporation and transpiration. Likewise, water from the atmosphere and groundwater can recharge surface waters. Atmospheric water falls as precipitation: rain, sleet, hail and snow. Groundwater that moves to the earth's surface contributes to the base flow of streams, lakes, wetlands and other waterways.
Precipitation initially infiltrates the top layers of the soil. Continuing precipitation may saturate the upper few inches of the soil, temporarily exceeding its capacity to hold water. Water accumulates on the land surface and moves to lower elevations through surface runoff and may occur across a small or large area.
A surface water system is characterized by its watershed or drainage basin. A watershed is the area of land draining to a specific river; the boundary is defined by the region's topography. Missouri's watersheds vary in size and can be nested within other larger watersheds. Land use within a watershed largely determines the quality of the local surface water. The quality of water leaving a watershed can, in turn, affect the cumulative quality of water far downstream. For example, pesticides detected in a city's drinking water supply could come from lawn and other urban uses or from an upstream watershed where agriculture is predominant. The previously discussed fate processes affect the levels of contaminants found in surface water.
Protecting water sources
It is difficult to clean water once it has become contaminated. Treatment is complicated, time-consuming, expensive, and often not feasible. The best solution to water contamination is to prevent it in the first place. The following pest management and pesticide handling practices can reduce the potential for contamination.
Practice integrated pest management
Pesticide application should be timed carefully and combined with other pest management practices. Pests should be identified accurately and pesticide applications made only when necessary, using the least amount needed for adequate pest control. Minimizing pesticide use cuts expenses and reduces potential for environmental problems.
Select pesticides carefully
Those pesticides that are not adsorbed to soil particles, are highly water soluble, and are relatively stable have the greatest potential to leach through the soil. Read pesticide labels carefully for information and restrictions about the rate, timing, and placement of the pesticide in that container. These factors affect the potential for leaching. Also note any water advisories or other water protection guidelines on the label.
Consider the vulnerability of the area
Determine how susceptible soil is to leaching. Soil texture, organic matter content, and permeability affect pesticide movement. Determine as accurately as possible the water table depth and the relative permeability of the geologic layers between the soil surface and the groundwater. Sinkholes can be especially troublesome because they allow surface water to reach groundwater quickly with little natural soil filtering.
Consider the location and condition of wells
Wells should be properly capped and sealed to prevent groundwater contamination. Grade the area around a wellhead to keep runoff away from the well. Pesticides spilled near wells can move directly and rapidly into groundwater. Some recommendations advise against mixing, storing, or disposing of pesticides within 100 feet of a well. Properly close all abandoned wells, and never dispose of wastes in unused wells.
Measure accurately
Carefully calculate how much pesticide concentrate is needed to treat the specific site with the equipment being used. Careful calculations not only can save money by reducing the amount of pesticide used, but can help eliminate disposal problems associated with excess spray mix.
Calibrate accurately
Calibrate equipment carefully and often to be certain the proper amount of pesticide will be applied. Check the equipment for leaks and malfunctions to minimize the potential for accidents or spills.
Mix and load carefully
Handle pesticides carefully to avoid spills. Mix and load pesticides on a concrete surface to avoid saturating the soil. Fill the spray tank as far from the water source as possible. Increase the length of the water hose or fill the tank in the field using an alternative water source. Never leave a spray unit unattended when filling.
Prevent back-siphoning
To prevent pesticides from back-siphoning into the water supply, keep the end of the fill hose above the water level in the spray tank. Use an anti-backflow device (check valve) on the fill hose, especially when siphoning water directly from a pond or stream. Proper well construction includes check-valves to prevent back-siphoning; check-valves can be added to an existing system.
Consider weather and irrigation
If you suspect heavy or sustained rain, delay the pesticide application. Runoff and leaching are favored by rainfall soon after application. The quality of irrigation water should be carefully controlled to minimize the potential for pesticide leaching and runoff.
Store pesticides safely
Minimize your pesticide inventory by buying only what is needed for a season or a specific spray job. The storage area should be away from all water sources. A concrete floor sealed with an impervious material eases cleanup in case of a spill or leak. Inspect containers regularly for leaks and corrosion. Bulk pesticide storage tanks should be inspected frequently and placed on concrete pads with dikes built around them to prevent the movement of pesticides if there is a spill or leak.
Dispose of wastes carefully
Follow all label instructions and restrictions when disposing of pesticides. Triple rinse or pressure rinse containers as soon as they are emptied and pour the rinsate into the spray tank. Excess spray mix and rinsates from equipment cleaning can be sprayed on another site or crop listed on the label. A source of water at the application site makes it easier to rinse equipment and to spray rinsates in the field. Where practical, excess spray mix or rinsates can be held in a tank for use in later spray mix.
Never dispose of pesticides or pesticide containers near a water source, over shallow water tables, in sinkholes or in abandoned wells
Excess pesticide concentrates can be given to another qualified user, safely stored until there is a hazardous waste collection day, or disposed of through a hazardous waste transporter.
Prevent spills
If a spill does occur, it should be contained and cleaned up immediately. Repeated pesticide spills in the same area can exceed the capacity of the soil to adsorb or degrade the chemical and can increase the likelihood of groundwater contamination.
Leave buffer zones around sensitive areas
When mixing, applying, storing or disposing (including cleanup) of pesticides, be aware of sensitive areas. These include springs, streams, ponds, wetlands and other surface waters; wells and groundwater recharge areas; and sinkholes. Establishing vegetation and leaving an untreated border are two ways to provide a buffer zone between sensitive areas and pesticide use or handling sites.
The fate of a pesticide and the likelihood of a pesticide moving into water are affected by the pesticide's chemical and physical properties, soil and geologic characteristics, climatic conditions, and pesticide handling practices. Each factor must be considered when determining the susceptibility of water to pesticide contamination.
Missouri aquifers and surface water supplies currently provide a vast supply of water for use in agriculture, homes and industry. They can remain a source of high-quality water for future needs only if they are protected now. Be sure to understand how your activities, including your handling and use of pesticides, may affect them. Seek assistance if you have questions or problems.
Checklist for protecting water from pesticides
- Store pesticides in their original containers in a cool, well-ventilated building with a concrete floor.
- Clean your pesticide application equipment in a way that makes it easy to collect rinsates.
- Install a check-valve on your water hose to prevent back-siphoning.
- Grade the area around your well to divert surface runoff.
- Ensure that any abandoned well near a pesticide handling or application site is properly closed.
- Build dikes around your bulk tanks to prevent off-site movement of pesticides.
- Know which pesticides you use have a potential for leaching.
- Delay pesticide applications if rain is forecast.
- Always check pesticide labels to learn irrigation practices, rates and application methods.
- Leave a border of untreated vegetation between treated and sensitive areas.
- Be aware of the geology and the relative depth of the groundwater in your area.
- Use pesticides only when necessary and then at the lowest rate needed to control a pest.
This publication is adapted from The Fate of Pesticides in the Environment and Groundwater Protection, Extension Agrichemical Fact Sheet Number 8, Pennsylvania State University.